Türkiye İlaç Piyasasında Dijital İlaçlar Kullanılabilecek mi?
22 Aralık 2017
İlaç Takip Çözümlerinin Getirileri ve Karşısındaki Engeller
29 Aralık 2017Drug track and trace systems are getting more and more popular in the pharmaceutical market. The most important effect of using track and trace systems is to increase productivity in production-sales channels and to reduce theft and fraud rates.
Increasing Productivity
With track and trace systems, there is an environment that supports competition for drug manufacturers. With the information obtained by the systems, it is possible to carry out studies that will increase sales and productivity in pharmaceutical production.
Ensuring Serialization and Traceability
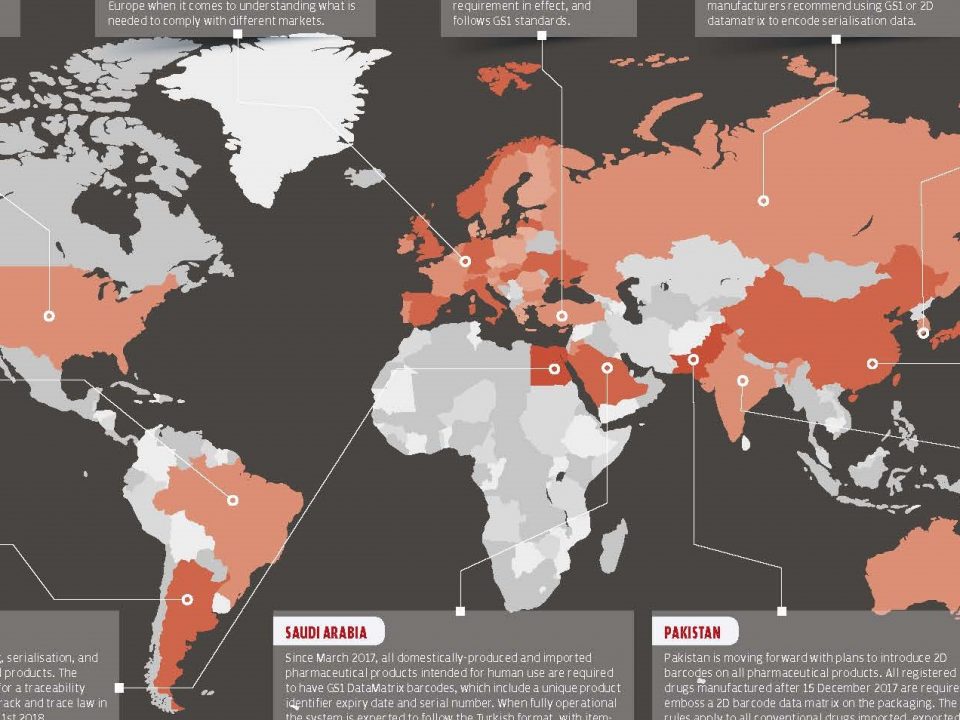
In the US and EU, Companies have gone one step further than serialization and have started to develop track and trace solutions using Data Matrix, RFID and QR code. Drug flow between production and sales channels can be monitored with these systems. All the manufacturers have to implement some kind of track and trace solution to their production line until the 2019.
War With the Counterfeit and Illicit Drugs
Drug manufacturers can not fight the counterfeit and illicit drugs alone. With investments of government and companies in track and trace solutions, counterfeit and illicit drugs can be identified. Market penetration of counterfeit drugs will be prevented from the beginning.
Although drug track and trace systems have been able to provide traceability and increase productivity with the information registered in these systems, they have not been able to be used everywhere in the world because of the obstacles that are present today.
OBSTACLES
No Common Standards
At the very least, the inability to establish a national standard is the greatest obstacle to serialization and the implementation of drug track and trace solutions.
In the US, the Food and Drug Administration (FDA) has asked the pharmaceutical industry to set a general standard for track and trace all drugs. The idea of establishing a common standard has arisen from the desire to assemble all the industry leaders under a common roof, facilitating their adaptation.

Safety Concerns
Some drug companies argue that the barcode system is not secure and is easily changeable. Apart from this, due to the presence of important personal information, there is concern that the track and trace system will be used by unauthorized people to access the information.

Slowing the Production Lines
Manufacturer thinks that the barcoding systems which is a part of the track and trace system, will slow down the production line and have increasing costs. This belief has made the implementation process difficult.

To resolve these concerns, track and trace systems should be studied in detail and every country should establish their standards according to their processes.
How a Good Track & Trace System Should Be?
If a track and trace system is established with the support of government and have a strong hierarchical order then we can say it is a good track and trace system. In such systems, if there is a breach in the one area, system will block only that area to prevent illegal actions and fix the problems.
For a good track and trace system, safety measures should be taken and these safety measures should have security levels. Security levels must be separated as physical and logical and logical security level must consist of different levels of difficulty within itself.
The track and trace system must have an advanced backup and recovery unit. Real-time data backup should be done in several places and be backed up from a second data center in case of a possible crash.