Sahte İlaçlar: Avrupa Birliğindeki Gelişmeler
11 Eylül 2017
Tedarik Zinciri Güvenliği ve İzlenebilirlik
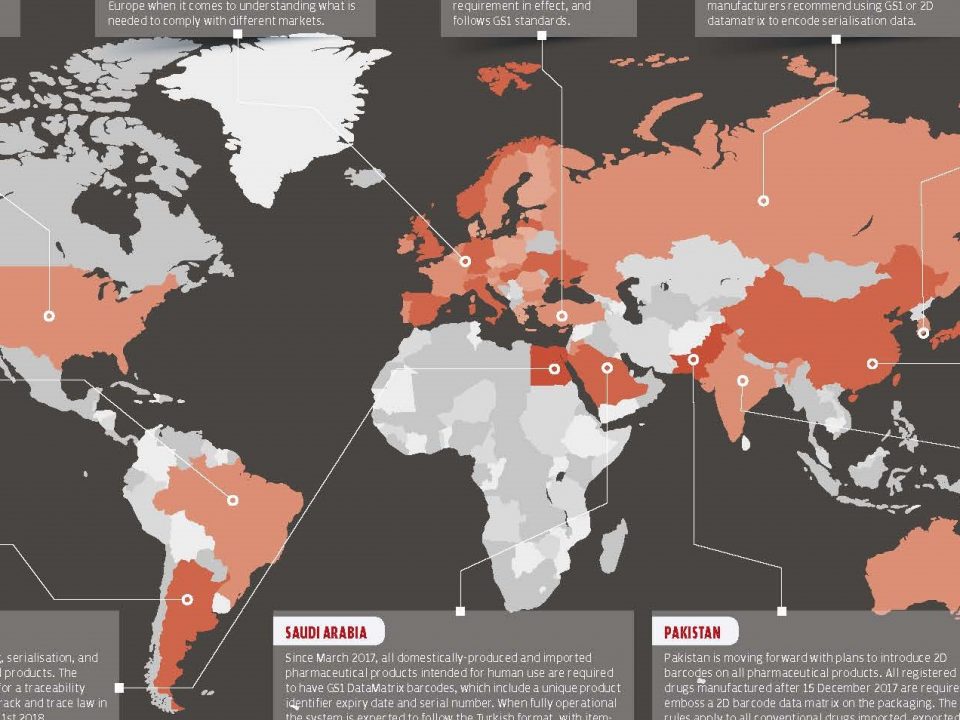
18 Eylül 2017No matter how advanced, no country has fully recovered from the problems associated with counterfeit medicines. Counterfeit medicine levels were low as most countries have regulatory systems and market controls. However, an increase in the number of counterfeit medicines has also started to take place in developed countries after a change in the market.
In an analysis published by the World Health Organization, noted that counterfeiting is higher at places where regulatory and legal oversight is weak.*
The anti-counterfeiting department has developed a multi-faceted strategy to fight against counterfeit medicines. Developed strategy includes;
- Gathering and analyzing information
- Working with national authorities with the skills and experience with this topic
- Working with organizations such as the World Customs Organization, the World Health Organization, Interpol and the World Intellectual Property Organization
- Ensuring that necessary arrangements are made in accordance with Directive 2011/62/EU.
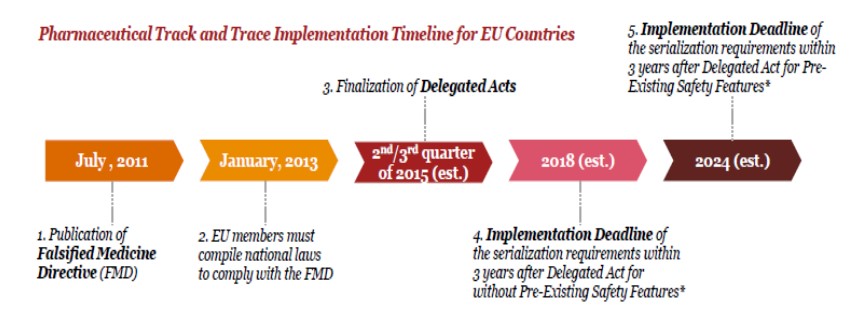
According to the timeline;
By 2018, serialization regulations have to be completed and all shareholders have to start using the given system. **
Following the work on the “Impact Assessment of Security Measures” published by the EU Commission, the following three items draw attention:
1. The unit product to be issued will have a 2D barcode contains manufacturer code, serial number, Global trade item number (GTIN), LOT number and expiration date. This special code will be the same all across the EU.
2. The medicine’s originality will be checked before being given to the end user. Medicines with a high risk of counterfeiting will also be subject to additional validation.
3. Special code should be managed by shareholders and national regulatory agencies should have system access to control and manage the registered data.
**pwc 2015 report, Turkish track and trace system