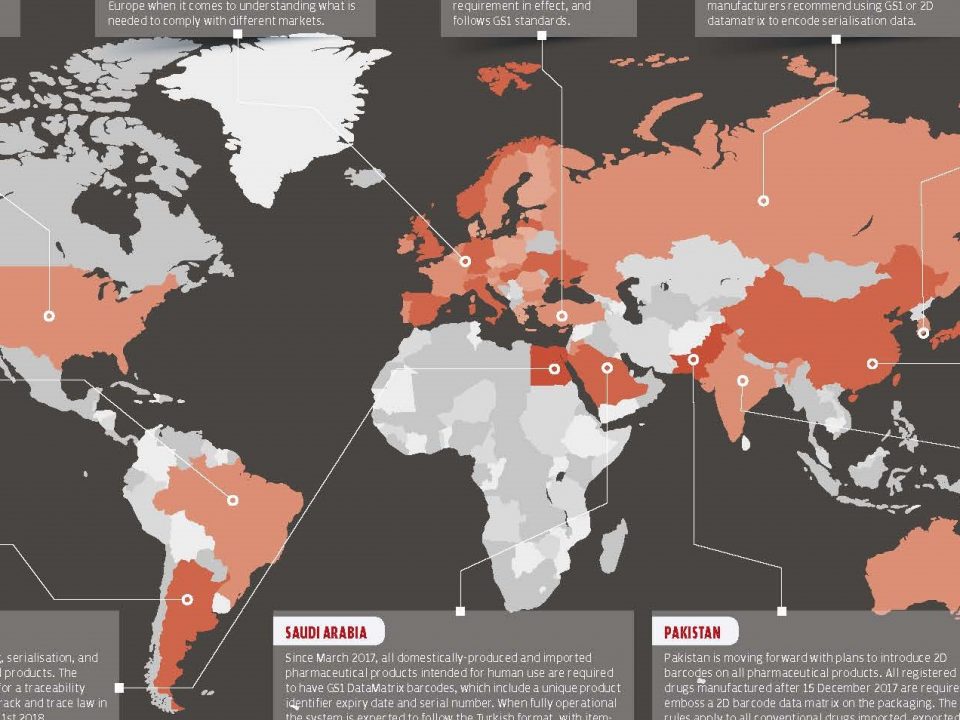
8. Akılcı İlaç Kullanımı İl Koordinatörleri Değerlendirme Toplantıları
21 Kasım 2017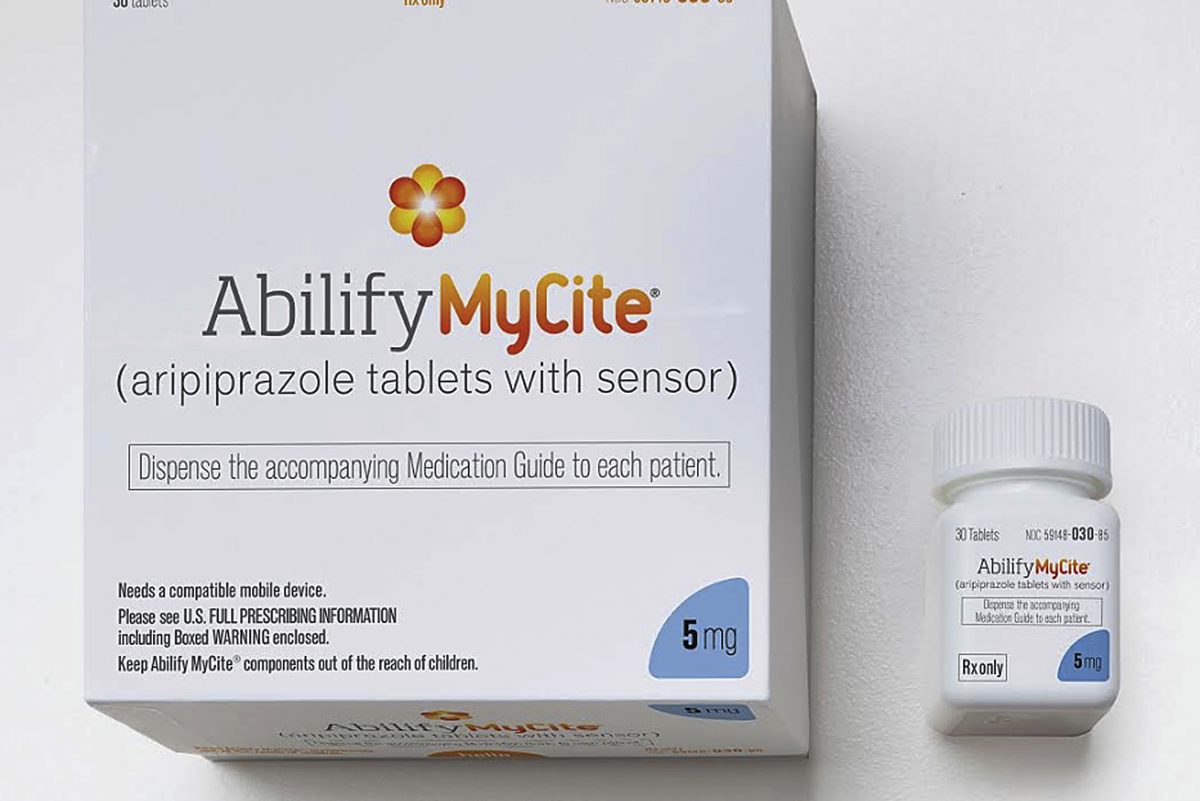
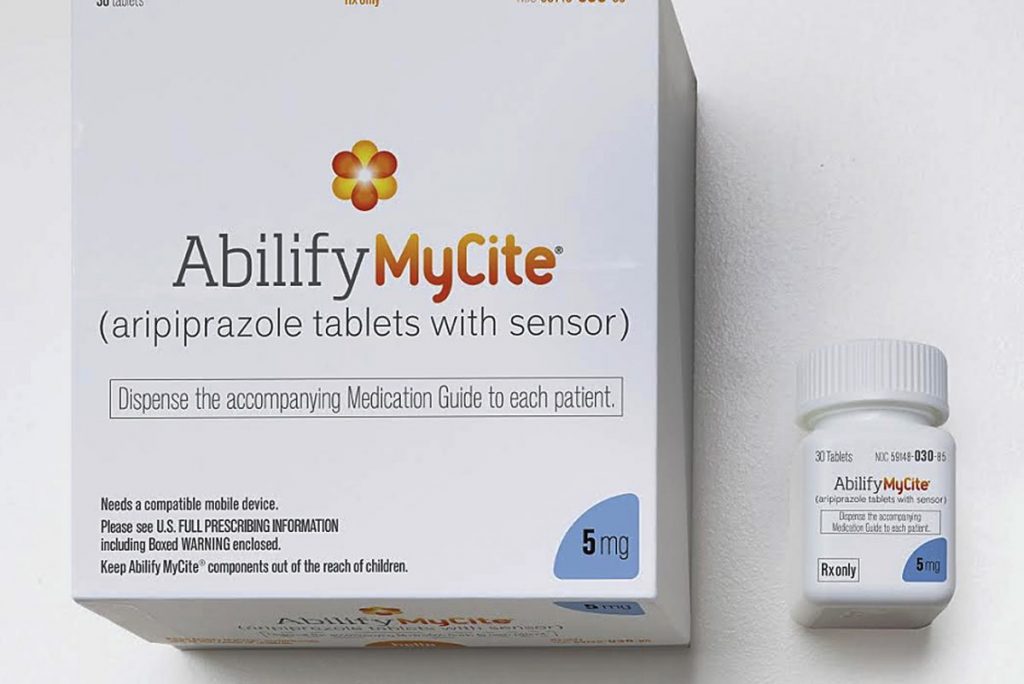
FDA İlk Takip Edilebilir Dijital İlacı Onayladı
27 Kasım 2017FDA approved the first digital drug, Abilify MyCite on 14 November 2017 with a press release.
The first digital drug, Abilify MyCite, was produced by Otsuka Pharma to track aripiprazole which is for the treatment of schizophrenia, bipolar disorder and depression in adults.
The sensor used in the medicine was made by Proteus firm.
The digital drug, is made by placing a small sensor in the tablet as small as a sand grain, stimulates the sensor by interacting with the gastric fluid in the stomach and records the date and time the drug is taken. The sensor sends the information to patch which the patient wears. The patch sends the data to a mobile application. The data then can be shared on chosen family member’s or/and caregiver’s phone.
Mitchell Mathis, director of the FDA Center of Drug Evaluation and Research for Psychiatry Products, says the follow-up of the use of prescription drugs for mental illness will be useful for patients and doctors, and the FDA supports the development and application of new technologies for prescription medicines.*
*https://www.fda.gov/…/pressannouncements